Asymmetric synthesis of 12-hydroxyheptadecatrienoic acid and its 5,6-dihydro- and 14,15-dehydro-derivatives†
Abstract
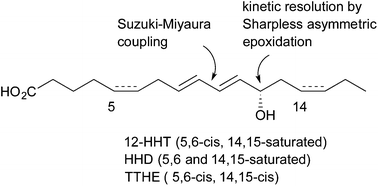
Natural 12-hydroxyheptadecatrienoic acid (12-HHT) with an S configuration was synthesised by a Suzuki–Miyaura coupling of C10–C17 iodo alcohol with C1–C9 vinylborane. The iodo alcohol was synthesised by utilising Sharpless asymmetric epoxidation of the corresponding trimethylsilyl alcohol. The method yielded more than 100 mg of 12-HHT. Similarly, syntheses of 5,6-dihydro- and 14,15-dehydro derivatives of 12-HHT, known as HHD and HHTE, respectively, were completed in a stereoselective manner.

 Please wait while we load your content...
Please wait while we load your content...