In situ generation of nitrilium from nitrile ylide and the subsequent Mumm rearrangement: copper-catalyzed synthesis of unsymmetrical diacylglycine esters†
Abstract
A novel in situ generation of nitrilium from a nitrile ylide and the subsequent Mumm rearrangement of carboxylic acid, nitrile, and diazo compounds gave various unsymmetrical diacylglycine esters in moderate to high yields. This copper-catalyzed cascade reaction enables one-pot generation of two C–N bonds, one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and one C–H bond, with nitrogen as the only byproduct. The reaction has a broad functional-group tolerance, is rapid, easily scales up to the 100 mmol scale, and is insensitive to air and moisture.
O bond, and one C–H bond, with nitrogen as the only byproduct. The reaction has a broad functional-group tolerance, is rapid, easily scales up to the 100 mmol scale, and is insensitive to air and moisture.
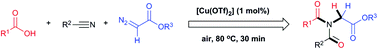

 Please wait while we load your content...
Please wait while we load your content...