Regioselective copper-catalyzed N(1)-(hetero)arylation of protected histidine†
Abstract
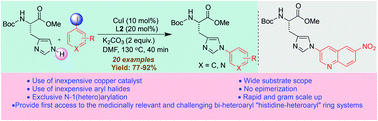
We report regioselective N(1)-arylation of protected histidine using copper(I) iodide as a catalyst, trans-N,N′-dimethylcyclohexane-1,2-diamine as a ligand and readily available aryl iodides as coupling partners under microwave irradiation at 130 °C for 40 min. The reaction provides rapid access to electron-donating, electron-withdrawing and bulky group substituted N-arylated histidines in high yields, including previously inaccessible N-heteroaryl histidines. These N(1)-(hetero)aryl histidines are promising building blocks in peptide-based drug design and discovery.

 Please wait while we load your content...
Please wait while we load your content...