De novo protecting-group-free total synthesis of (+)-muricadienin, (+)-ancepsenolide and (+)-3-hexadecyl-5-methylfuran-2(5H)-one†
Abstract
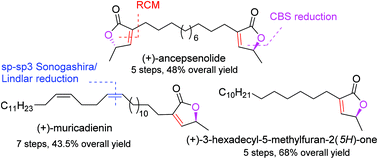
A de novo protecting-group-free total synthesis of (+)-muricadienin, (+)-ancepsenolide and (+)-3-hexadecyl-5-methylfuran-2(5H)-one has been achieved. Ring-closing-metathesis has been the key step in the synthesis. In (+)-muricadienin synthesis, a long chain alkyl group has been installed by an sp–sp3 Sonogashira type reaction followed by a cis-selective Lindlar reduction. The total synthesis is achieved in 7 steps and in excellent 43.5% overall yield. Similarly, (+)-ancepsenolide and (+)-3-hexadecyl-5-methylfuran-2(5H)-one synthesis is completed in 5 steps each and in 48 and 68% overall yields, respectively.

 Please wait while we load your content...
Please wait while we load your content...