Chemoselective photooxygenations of furans bearing unprotected amines: their use in alkaloid synthesis
Abstract
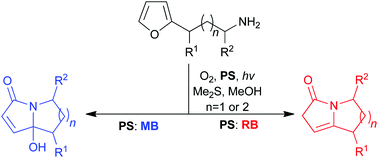
Very recent investigations are described, which have shown how basic and unprotected nitrogen functionalities can be included, problem-free, in the furan photooxidation step of singlet oxygen-initiated cascade reaction sequences. The amine groups do not react with singlet oxygen, but, instead, participate later on in the sequences that ultimately yield a diverse range of important alkaloid motifs. To illustrate the versatility of this chemistry, six natural products were synthesised very rapidly and efficiently. Furthermore, all the new technologies operated under green conditions and without the use of a single protecting group.

 Please wait while we load your content...
Please wait while we load your content...