PIFA-mediated ethoxyiodination of enamides with potassium iodide†
Abstract
The regioselective ethoxyiodination of enamides was developed using PIFA in combination with potassium iodide in ethanol. The reaction proceeds regioselectively with excellent yields and diastereoselectivities, providing valuable synthons for further functionalisations. Control experiments were conducted, indicating that the transformation occurs through an ionic manifold involving an in situ generated hypoiodite species.
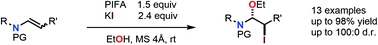


 Please wait while we load your content...
Please wait while we load your content...