C2-Symmetric pyrrolidine-derived squaramides as recyclable organocatalysts for asymmetric Michael reactions†
Abstract
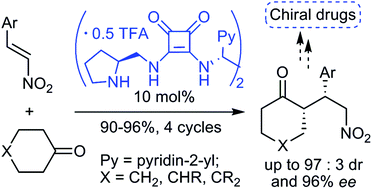
Novel C2-symmetric N,N′-bis-[(pyrrolidin-2-yl)methyl-squaramide] TFA salts bearing (R,R)- or (S,S)-1,2-di(pyridin-2-yl)ethane spacer groups were synthesized and applied, in combination with TEA, as efficient organocatalysts for asymmetric reactions between cyclohexanone derivatives and β-nitrostyrenes to afford the corresponding Michael adducts in high yields with good to excellent enantioselectivity. The catalytic procedure is readily scalable and recyclable over four times without a negative impact on the selectivity of the reaction. Some of the prepared compounds are valuable precursors to useful bioactive molecules.

 Please wait while we load your content...
Please wait while we load your content...