A toolset of functionalized porphyrins with different linker strategies for application in bioconjugation†
Abstract
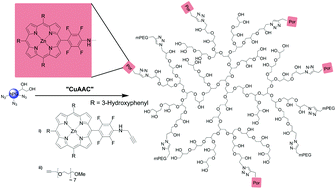
The reaction of amines with pentafluorophenyl-substituted A3B-porphyrins has been used to obtain different useful reactive groups for further functionalization and/or conjugation of these porphyrins to other substrates or materials. Porphyrins with alkenyl, alkynyl, amino, azido, epoxide, hydroxyl, and maleimido groups have thus been synthesized. For the first time such functionalized porphyrins have been conjugated to hyperbranched polyglycerol (hPG) as a biocompatible carrier system for photodynamic therapy (PDT) using the copper(I)-catalyzed 1,3-dipolar cycloaddition (CuAAC). The photocytotoxicity of selected porphyrins as well as of the porphyrin-hPG-conjugates has been assessed in cellular assays with human epidermoid carcinoma A-253 and squamous carcinoma CAL-27 cells. For several biomedical applications a release of the active drug and/or fluorescent dye is desired. Therefore, additionally, the synthesis of A3B-porphyrins with cleavable linker moieties is presented, namely disulfide, cleavable in a reductive environment, and acetal linkers whose cleavage is pH triggered.

 Please wait while we load your content...
Please wait while we load your content...