Organophosphorus-mediated N–N bond formation: facile access to 3-amino-2H-indazoles†
Abstract
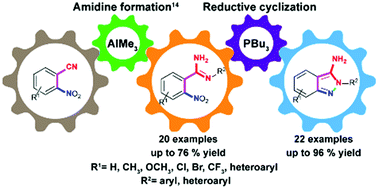
A convenient and efficient strategy has been devised to access 3-amino-2H-indazole derivatives in two steps from readily available starting materials. The conversion of 2-nitrobenzonitriles to substituted benzamidines followed by an organophosphorus-mediated reductive cyclization and a subsequent N–N bond formation afforded 3-amino-2H-indazoles in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...