Catching anions with coloured assemblies: binding of pH indicators by a giant-size polyammonium macrocycle for anion naked-eye recognition†
Abstract
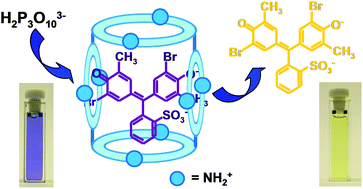
A giant-size polyamine macrocycle L, composed of four 1,4,8,11-tetraazacyclotetradecane (cyclam) units linked by 1,3-dimethylenbenzyl spacers, strongly interacts in aqueous solution with four pH indicators (bromocresol purple (H2BCP), phenol red (H2PR), phenolphthalein (H2PP) and fluorescein (H2F)) in their anionic forms. Besides 1 : 1 complexes, L also forms assemblies with an unusual 1 : 2 receptor to dye stoichiometry, thanks to its large dimensions, which allow for the simultaneous interaction of the receptor protonated forms with two anionic dyes. The formation of the assemblies markedly affects the pKa values of the phenol groups of the dyes, which change colour upon complexation in well-defined pH ranges. This property can be effectively exploited for optical detection of anions. The L-H2BCP 1 : 2 assembly is able to selectively detect the triphosphate anion at slightly acidic pH values, thanks to the release, upon triphosphate coordination, of the dye from the ensemble, with a consequent colour change of the solution from purple-violet (complexed BCP2− dye) to yellow (free BCP2−). No effect is caused by other inorganic anions. The L-H2BCP 1 : 2 assembly represents a rare case of an optical chemosensor for the triphosphate anion.


 Please wait while we load your content...
Please wait while we load your content...