A short and modular approach towards 3,5-disubstituted indolizidine alkaloids†
Abstract
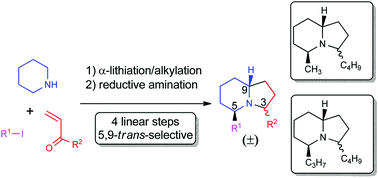
3,5-Dialkyl indolizidines have been prepared in four linear steps from commercially available starting materials. The sequence involves two direct α-functionalization steps and a subsequent reductive amination and provides diastereoselective access to both C-3 epimers of the 5,9-trans-substituted indolizines. The naturally occurring indolizidines 195B and 223AB have been synthesized using this methodology.

 Please wait while we load your content...
Please wait while we load your content...