Biosynthesis-driven structure–activity relationship study of premonensin-derivatives†
Abstract
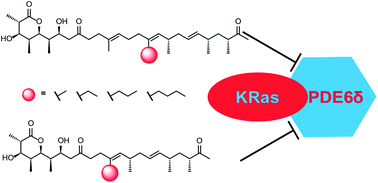
The controlled derivatization of natural products is of great importance for their use in drug discovery. The ideally rapid generation of compound libraries for structure–activity relationship studies is of particular concern. We here use modified biosynthesis for the generation of such a library of reduced polyketides to interfere with the oncogenic KRas pathway. The polyketide is derivatized via side chain alteration, and variations in its redox pattern and in its backbone chain length through manipulation in the corresponding polyketide synthase. Structural and biophysical analyses revealed the nature of the interaction between the polyketides and KRas-interacting protein PDE6δ. Non-natural polyketides with low nanomolar affinity to PDE6δ were identified.


 Please wait while we load your content...
Please wait while we load your content...