Iridium(iii)-catalyzed regioselective direct arylation of sp2 C–H bonds with diaryliodonium salts†
Abstract
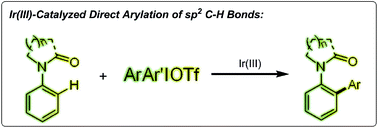
A regioselective direct arylation of arenes and olefins at the ortho position is reported. The key to the high selectivity is the appropriate choice of diaryliodonium salts as the arylating reagent in the presence of a cationic iridium(III) catalyst. The coordination of the metal with an oxygen atom or a nitrogen atom and subsequent C–H activation allows for direct arylation with coupling partners. This reaction proceeds under mild reaction conditions and with a high tolerance of various functional groups including many halide functional groups.

 Please wait while we load your content...
Please wait while we load your content...