Iodine-mediated synthesis of heterocycles via electrophilic cyclization of alkynes
Abstract
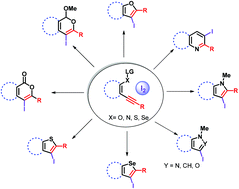
Iodine has been recognized as an efficient, non-toxic, readily available and easy-to-handle electrophilic reagent to favour halocyclization reactions for the synthesis of novel iodofunctionalized heterocyclic molecules that serve as versatile intermediates in synthetic organic chemistry. This review presents numerous useful methodologies for the synthesis of O, N, S, and Se-heterocycles through electrophilic cyclization via the attack of an electrophile on the C(sp) bond of alkynes. The cyclization proceeds under mild reaction conditions and tolerates a wide variety of functional groups.

 Please wait while we load your content...
Please wait while we load your content...