Synthesis of enantiopure cyclopropyl esters from (−)-levoglucosenone†
Abstract
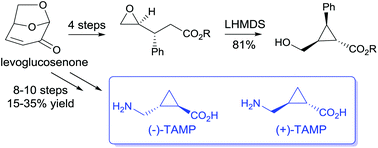
The biorenewable chiral synthon (−)-levoglucosenone has been converted to enantiopure cyclopropyl esters using the base-promoted carbocyclisation of 4,5-epoxyvalerates. This protocol was applied to the enantiospecific synthesis of the GABAc receptor agonist (1R,2R)-trans-2-aminomethylcyclopropanecarboxylic acid ((−)-TAMP) and its enantiomer. The process was also extended to generate 1,1,2- and 1,2,3-trisubstituted cyclopropanes resulting in a formal synthesis of the selective glutamate receptor antagonist PCCG-4.


 Please wait while we load your content...
Please wait while we load your content...