Protein ubiquitination via dehydroalanine: development and insights into the diastereoselective 1,4-addition step†
Abstract
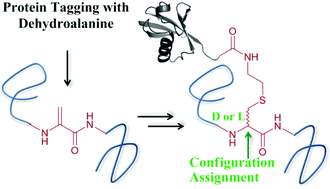
We report a strategy for site-specific protein ubiquitination using dehydroalanine (Dha) chemistry for the preparation of ubiquitin conjugates bearing a very close mimic of the native isopeptide bond. Our approach relies on the selective formation of Dha followed by conjugation with hexapeptide bearing a thiol handle derived from the C-terminal of ubiquitin. Subsequently, the resulting synthetic intermediate undergoes native chemical ligation with the complementary part of the ubiquitin polypeptide. It has been proposed that the Michael addition step could result in the formation of a diastereomeric mixture as a result of unselective protonation of the enolate intermediate. It has also been proposed that the chiral protein environment may influence such an addition step. In the protein context these questions remain open and no experimental evidence was provided as to how such a protein environment affects the diastereoselectivity of the addition step. As was previously proposed for the conjugation step on protein bearing Dha, the isopeptide bond formation step in our study resulted in the construction of two protein diastereomers. To assign the ratio of these diastereomers, trypsinization coupled with high-pressure liquid chromatography analysis were performed. Moreover, the obtained peptide diastereomers were compared with identical synthetic peptides having defined stereogenic centers, which enabled the determination of the configuration of the isopeptide mimic in each diastereomer. Our study, which offers a new method for isopeptide bond formation and protein ubiquitination, gives insights into the parameters that affect the stereoselectivity of the addition step to Dha for chemical protein modifications.
 Please wait while we load your content...
Please wait while we load your content...