Bis(arylmethyl)-substituted unsymmetrical phosphites for the synthesis of lipidated peptides via Staudinger-phosphite reactions†
Abstract
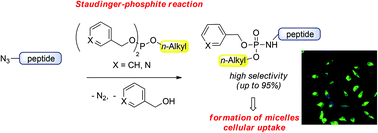
With this study we introduce new unsymmetrical phosphites to obtain lipidated peptide-conjugates starting from easily accessible azide-modified amino acid or peptide precursors. For this purpose, we investigated which substituents at alkyl phosphites lead to the highest formation of mono-alkylated phosphoramidate peptides. We found that phosphites containing one alkyl-chain and two picolyl or benzyl-substituents delivered alkyl phosphoramidate-conjugates in high yields, which also allowed a chemoselective lipidation of an unprotected azido polypeptide. Finally, monolipidated phosphoramidate peptides obtained by the unsymmetrical Staudinger phosphite reaction led to the formation of micelle-like structures and cellular uptake.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...