Efficient synthesis of longer Aβ peptides via removable backbone modification†
Abstract
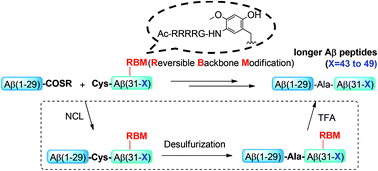
Longer amyloid-beta (Aβ) peptides (43 to 49 amino acids) play essential roles in the pathology of Alzheimer's disease (AD). The difficulty in the preparation of longer Aβ peptides is still an obstacle to elucidate their roles in AD. Herein we report a robust and efficient strategy for the chemical synthesis of longer Aβ peptides (Aβ48 and Aβ49). A key feature of this method is the installation of removable Arg4-tagged backbone modification groups into the hydrophobic region of Aβ. This modification can improve the handling properties of the purification, ligation and mass characterization of longer Aβ peptides. The practicability of the new method has been demonstrated by the successful synthesis of Aβ48 and Aβ49 peptides.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...