New potent αvβ3 integrin ligands based on azabicycloalkane (γ,α)-dipeptide mimics†
Abstract
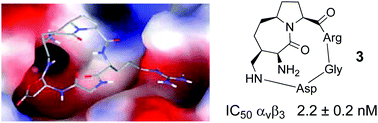
We have designed a new synthetic strategy for the preparation of a new class of cyclic RGD integrin ligands in which the azabicycloalkane scaffold can be envisaged as a (γ,α) dipeptide mimic. The synthesis and in vitro biological evaluation of these RGD derivatives, as well as the computational study of their conformational properties and binding modes to αVβ3 integrin are described. Compound 3 has shown to be a promising candidate as αVβ3 integrin antagonist able to interfere with both cell adhesion and movement on vitronectin with no evidence of cytotoxic effects.

 Please wait while we load your content...
Please wait while we load your content...