Rationalization of the selectivity between 1,3- and 1,2-migration: a DFT study on gold(i)-catalyzed propargylic ester rearrangement†
Abstract
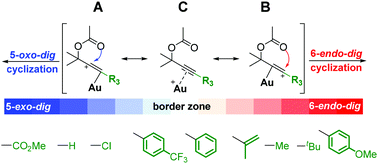
Gold catalyzed rearrangement of propargylic esters can undergo 1,3-acyloxy migration to form allenes, or undergo 1,2-acyloxy migration to access gold-carbenoids. The variation in migration leads to different reactivities and diverse cascade transformations. The effect of terminal substituents is very important for the rearrangement. However, it remains ambiguous how terminal substituents govern the selectivity of the rearrangement. This study presents a theoretical model based on the resonance structure of gold activated propargylic ester complexes to rationalize the rearrangement selectivity. Substrates with a major resonance contributor A prefer 5-exo-dig cyclization (1,2-migration), while those with a major resonance contributor B prefer 6-endo-dig cyclization (1,3-migration). This concise model would be helpful in understanding and tuning the selectivity of the metal catalyzed rearrangement of propargylic esters.

 Please wait while we load your content...
Please wait while we load your content...