Aminoquinoline-assisted vinylic C–H arylation of unsubstituted acrylamide for the selective synthesis of Z olefins†
Abstract
A method for Pd-catalyzed, aminoquinoline-directed arylation of vinylic C–H bonds with aryl iodides has been developed. This reaction represents a rare example of Pd-catalyzed vinylic C–H functionalization of unsubstituted acrylamide, allowing for the highly regio- and stereoselective preparation of Z-olefins. High tolerance to functional groups is observed with good yields and excellent selectivity. It offers a complementary synthetic method to traditional pathways for Z-olefins.
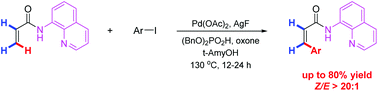


 Please wait while we load your content...
Please wait while we load your content...