Artificial supramolecular protein assemblies as functional high-order protein scaffolds
Abstract
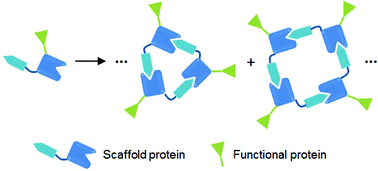
Supramolecular assemblies of protein building blocks potentially offer unique biomaterials with unmatched functionalities as well as atomic level structural accuracy. An increasing number of assembling strategies have been reported for the fabrication of diverse artificial protein assemblies, ranging from rather heterogeneous protein oligomers to computationally designed discrete protein architectures. In this perspective, we discuss these artificial protein supramolecules in terms of their use as highly potent high-order protein scaffolds that can display various functional proteins with precise structural and valency control. Following a brief overview of current approaches for protein assembly, several examples of functional protein assemblies have been introduced, with a particular focus on our recent report of valency-controlled green fluorescent protein nano-assemblies. Our supramolecular protein scaffolds allow building a series of polygonal assemblies of functional binding proteins, which provide unprecedented ways to study multivalent protein interactions. Even with many remaining challenges, there is unlimited potential of artificial protein scaffolds in many fields from nanotechnology to vaccine development.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...