Synthesis of highly functionalized oligobenzamide proteomimetic foldamers by late stage introduction of sensitive groups†
Abstract
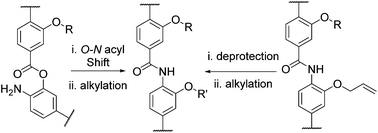
α-Helix proteomimetics represent an emerging class of ligands that can be used to inhibit an array of helix mediated protein–protein interactions. Within this class of inhibitor, aromatic oligobenzamide foldamers have been widely and successfully used. This manuscript describes alternative syntheses of these compounds that can be used to access mimetics that are challenging to synthesize using previously described methodologies, permitting access to compounds functionalized with multiple sensitive side chains and accelerated library assembly through late stage derivatisation.

 Please wait while we load your content...
Please wait while we load your content...