I2 mediated synthesis of 5-substituted-3-methyl/benzyl-1,3,4-oxadiazol-2(3H)-ones via sequential condensation/oxidative cyclization and rearrangement†
Abstract
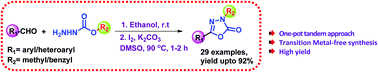
A simple and efficient iodine-assisted protocol for the synthesis of 5-substituted-3-methyl/benzyl-1,3,4-oxadiazol-2(3H)-ones has been developed. The reaction involves a sequential condensation followed by tandem oxidative cyclization and rearrangement of readily available methyl/benzyl carbazates and aldehydes as starting substrates. The presence of iodine and base promotes intramolecular C–O bond formation, followed by Chapman-like rearrangement at 90 °C of the methyl/benzyl group in the hydrazone intermediate formed during the condensation step. This transition-metal-free approach has been adopted to generate a variety of oxadiazolones under mild conditions in good to excellent yields.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...