Solvatofluorochromic, non-centrosymmetric π-expanded diketopyrrolopyrrole†
Abstract
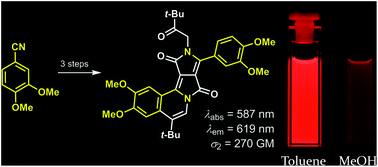
A novel non-centrosymmetric π-expanded diketopyrrolopyrrole was designed and synthesized. Strategic placement of tert-butyl groups at the periphery of a diketopyrrolopyrrole allowed us to selectively fuse one moiety via tandem Friedel–Crafts-dehydration reactions, resulting in a non-centrosymmetric dye. The structure of the dye was confirmed by X-ray crystallography, revealing that it contains a nearly flat arrangement of four fused rings. Extensive photophysical studies of this new functional dye revealed that the intensity of its emission strongly depends on solvent polarity, which is typical for dipolar chromophores. In non-polar solvents, the fluorescence quantum yield is high whereas in polar solvents such as MeOH, it is 12%. However, upon two-photon excitation the compound behaves like a centrosymmetric dye, showing a two-photon absorption maximum at significantly shorter wavelengths than twice the wavelength of the one-photon absorption maximum.

 Please wait while we load your content...
Please wait while we load your content...