Design, synthesis, and duplex-stabilizing properties of conformationally constrained tricyclic analogues of LNA†
Abstract
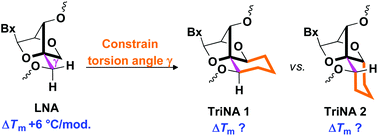
The design, synthesis and biophysical evaluation of two highly-constrained tricyclic analogues of locked nucleic acid (LNA), which restrict rotation around the C4′–C5′-exocyclic bond (torsion angle γ) and enhance hydrophobicity in the minor groove and along the major groove, are reported. A structural model that provides insights into the sugar–phosphate backbone conformations required for efficient hybridization to complementary nucleic acids is also presented.

 Please wait while we load your content...
Please wait while we load your content...