An approach to “escape from flatland”: chemo-enzymatic synthesis and biological profiling of a library of bridged bicyclic compounds†
Abstract
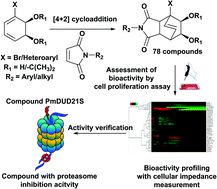
A major reason for the low success rate in current drug development through chemical synthesis has been ascribed to the large fraction of quasi planar candidate molecules. Therefore, an “escape from flatland” strategy has been recommended for the generation of bioactive chemical entities. In a first attempt to test this recommendation, we synthesized a small collection of bridged bicyclic compounds possessing a rigid spherical core structure by combining a group of cyclic dienes with a collection of dienophiles. We started from planar biphenyl analogues and, by enzymatic dioxygenation, transformed them into hydroxylated diene structures. Using a small library of newly synthesized dienophiles, the dienes were converted into bridged bicycles via the Diels–Alder reaction. The resulting collection of 78 structures was first tested for bioactivity in a generic assay based on interference with the proliferation of mammalian cells. A more mechanism-targeted bioactivity profiling method, exploiting cellular impedance monitoring, was subsequently used to obtain suggestions for the mode of action exerted by those compounds that were the most active in the proliferation assay. Proteasome inhibition could be confirmed for 8 of a series of 9 respective candidates. Whilst 7 of these molecules showed relatively weak interference with proteasome activity, one candidate exerted a moderate but distinct inhibition. This result appears remarkable in view of the small size of the compound library, which was synthesized following a few basic considerations. It encourages the application of diverse synthetic approaches to further investigate the role of spherical shape for the success of compound libraries.

 Please wait while we load your content...
Please wait while we load your content...