Solution-phase synthesis and biological evaluation of triostin A and its analogues†
Abstract
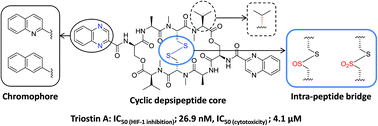
Triostin A is a biosynthetic precursor of echinomycin which is one of the most potent hypoxia inducible factor 1 (HIF-1) inhibitors. An improved solution-phase synthesis of triostin A on a preparative scale has been achieved in 17.5% total yield in 13 steps. New analogues of triostin A with various aromatic chromophores, oxidized intra-peptide disulfide bridges and diastereoisomeric cyclic depsipeptide cores were also successfully synthesized. All analogues had a significant inhibitory effect on HIF-1 transcriptional activation in hypoxia and cytotoxicity on MCF-7 cells, with the exception of the derivatives containing a naphthalene chromophore or a thiosulfonate bridge. For the first time, triostin A, echinomycin and the thiosulfinate analogue of triostin A have been revealed to inhibit not only DNA binding of HIF-1 but also HIF-1α protein accumulation in MCF-7 cells. Furthermore, the thiosulfinate analogue and triostin A exhibited a hypoxia-selective cytotoxicity on MCF-7 cells. The improved solution-phase synthetic procedure described herein will contribute to the development of diverse bicyclic depsipeptide drug candidates with the potential to act as novel anti-cancer agents targeting hypoxic tumor microenvironments.

 Please wait while we load your content...
Please wait while we load your content...