Room-temperature cobalt-catalyzed arylation of aromatic acids: overriding the ortho-selectivity via the oxidative assembly of carboxylate and aryl titanate reagents using oxygen†
Abstract
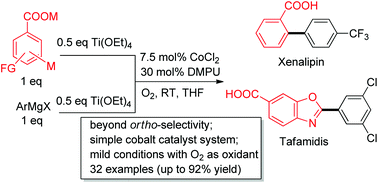
A room temperature phosphine or NHC ligand-free cobalt-catalyzed arylation of (hetero)aromatic acids has been developed. It involves an oxidative cross-coupling between carboxylate and aryl titanate reagents using oxygen as an oxidant, and the arylation at the position ortho, meta and para to the carboxylic acid group could all be achieved. As application, various (hetero)aromatic acids including xenalipin, tafamidis and the key intermediate for a cardioprotective compound have been efficiently synthesized.

 Please wait while we load your content...
Please wait while we load your content...