Iodine mediated intramolecular C2-amidative cyclization of indoles: a facile access to indole fused tetracycles†
Abstract
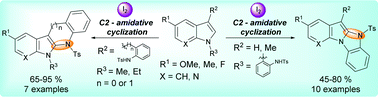
A novel and metal-free I2-mediated intramolecular C2 amidation of indoles under mild reaction conditions is developed. This methodology affords various indole fused tetracyclic compounds, such as benzo[4,5]imidazo[1,2-a]indoles by intramolecular C2 amidation of N-aryl substituted indoles. This C2 sulfonamidative cyclization also offers convenient access to indolo[2,3-b]indoles and dihydroindolo[2,3-b]quinoline from C3 aryl substituted indoles in good to excellent yields. Indolo[2,3-b]quinolines are also synthesized by the domino cyclization–detosylation–aromatization reaction sequence.

 Please wait while we load your content...
Please wait while we load your content...