Influence of the oxazole ring connection on the fluorescence of oxazoyl-triphenylamine biphotonic DNA probes†
Abstract
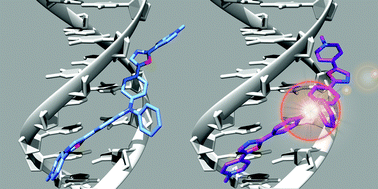
On the basis of our previous work on DNA fluorophores derived from vinylpyridinium-triphenylamine, we explored the structure space around the electron-rich triphenylamine (TP) core by changing the vinyl bond to an oxazole ring. As 2,5-diaryloxazoles are known to be highly fluorescent and efficient two photon absorbers, we synthesized analogues with two different connections of the oxazole to the triphenylamine core: TP-Ox2Py and TP-Ox5Py sets. Since the benzimidazolium group was proven to be more effective in the TP series than the pyridinium, we also synthesized a TP-Ox5Bzim set. The TP-Ox5Py series retains the TP-Py properties: on/off behavior on DNA, good two-photon cross-section and bright staining of nuclear DNA by microscopy under both one or two-photon excitation. On the other hand, the TP-Ox2Py series does not display fluorescence upon binding to DNA. The TP-Ox5Bzim set is fluorescent even in the absence of DNA and displays lower affinity than the corresponding TP-Ox5Py. CD experiments and docking were performed to understand these different behaviors.

 Please wait while we load your content...
Please wait while we load your content...