An economical synthesis of substituted quinoline-2-carboxylates through the potassium persulfate-mediated cross-dehydrogenative coupling of N-aryl glycine derivatives with olefins†
Abstract
A practical and economical K2S2O8-mediated oxidative cross-dehydrogenative coupling of N-aryl glycine derivatives with olefins has been established, affording structurally diverse quinoline-2-carboxylates in good to high efficiency. The low cost, negligible toxicity, and ease of handling of K2S2O8 combined with the absence of hazardous byproducts and the easy workup consisting of simple filtration are attractive based on economic and environmental factors.
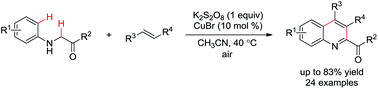

 Please wait while we load your content...
Please wait while we load your content...