A novel C,D-spirodioxene taxoid synthesized through an unexpected Pd-mediated ring cyclization†
Abstract
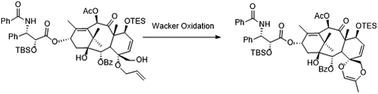
A novel C,D-spirodioxene taxoid (6) was prepared from paclitaxel (1a), with the key steps including an unexpected Pd-mediated ring cyclization. The anti-tubulin activity of 6 was decreased relative to that of 1a and a previously reported C,D-spirolactone taxane (5). These observations could be rationalized on the basis of molecular modeling results. To the best of our knowledge, this is the first example indicating that 1,4-dioxenes can be synthesized from a mono-allyl vicinal diol through a Wacker-type cyclization. This strategy may be applicable to the synthesis of other C,D-spiro taxoids.

 Please wait while we load your content...
Please wait while we load your content...