A new suprasterol by photochemical reaction of 1α,25-dihydroxy-9-methylene-19-norvitamin D3†
Abstract
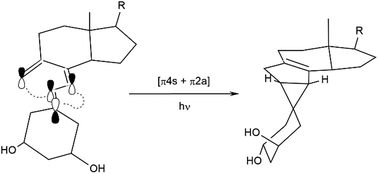
The UV-induced photochemical reaction of 1α,25-dihydroxy-9-methylene-19-norvitamin D3 has been investigated. The pentacyclic structure of the isolated product has been unequivocally established by X-ray crystallographic analysis. The possible reaction paths of the examined photochemical transformation are discussed. Biological in vivo and in vitro tests proved that the photoproduct is devoid of calcemic activity.


 Please wait while we load your content...
Please wait while we load your content...