anti-Selective aminofluorination of alkenes with amidines mediated by hypervalent iodine(iii) reagents†
Abstract
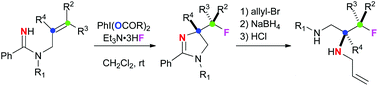
anti-Selective aminofluorination of alkenes with amidines was enabled by hypervalent iodine(III) reagents, affording 4-fluoroalkyl-2-imidazolines. Further reductive ring-opening of the 2-imidazoline moiety could deliver highly functionalized 3-fluoropropane-1,2-diamine derivatives.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...