Electrochemical phase formation: classical and atomistic theoretical models
Abstract
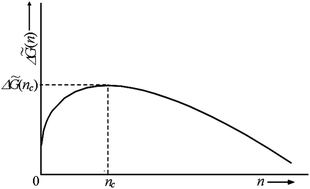
The process of electrochemical phase formation at constant thermodynamic supersaturation is considered in terms of classical and atomistic nucleation theories. General theoretical expressions are derived for important thermodynamic and kinetic quantities commenting also upon the correlation between the existing theoretical models and experimental results. Progressive and instantaneous nucleation and growth of multiple clusters of the new phase are briefly considered, too.
- This article is part of the themed collection: Electrochemical processes at the nanoscale: from fundamentals to applications

 Please wait while we load your content...
Please wait while we load your content...