Partially oxidized iridium clusters within dendrimers: size-controlled synthesis and selective hydrogenation of 2-nitrobenzaldehyde†
Abstract
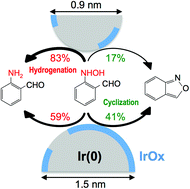
Iridium clusters nominally composed of 15, 30 or 60 atoms were size-selectively synthesized within OH-terminated poly(amidoamine) dendrimers of generation 6. Spectroscopic characterization revealed that the Ir clusters were partially oxidized. All the Ir clusters efficiently converted 2-nitrobenzaldehyde to anthranil and 2-aminobenzaldehyde under atmospheric hydrogen at room temperature in toluene via selective hydrogenation of the NO2 group. The selectivity toward 2-aminobenzaldehyde over anthranil was improved with the reduction of the cluster size. The improved selectivity is ascribed to more efficient reduction than intramolecular heterocyclization of a hydroxylamine intermediate on smaller clusters that have a higher Ir(0)-phase population on the surface.

 Please wait while we load your content...
Please wait while we load your content...