Electrochemical de-alloying in two dimensions: role of the local atomic environment
Abstract
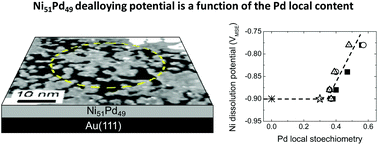
We investigate by in situ scanning tunnelling microscopy (STM) the potential dependence of the electrochemical dealloying of NiPd monoatomic layers electrodeposited on Au(111). The dealloying process is achieved by Ni selective dissolution and was studied as a function of NiPd composition: for an alloy with a Ni content ≥70%, quasi-complete Ni dissolution is achieved at a potential of −0.9 VMSE whereas for a Ni content <70%, Ni dissolution at the same potential drastically slows down after the removal of small amounts of Ni. The alloy morphology at this “passivation state” is characterized by the presence of holes in the alloy monolayer with evidence for the Pd enrichment at the hole edges. These findings are confirmed by Monte Carlo simulations. Further Ni dissolution at passivation was achieved by applying more positive potentials which depend on the alloy composition. These results allowed us to determine the correlation between the Ni dissolution onset potential and the local Pd content.
- This article is part of the themed collection: Electrochemical processes at the nanoscale: from fundamentals to applications

 Please wait while we load your content...
Please wait while we load your content...