Electrochemical metallization switching with a platinum group metal in different oxides†
Abstract
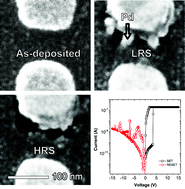
In a normal electrochemical metallization (ECM) switch, electrochemically active metals, such as Ag and Cu are used to provide mobile ions for the conducting filament. In both ECM and valence change memory (VCM) devices, platinum group metals, such as Pt and Pd, are typically used as the counter electrode and assumed to be chemically and physically inert. In this study, we explore whether the so-called inert metal itself can form a conducting filament and result in repeatable resistance switching. Pd and different oxide host matrices are used for this purpose. We have observed that the transport of oxygen anions dominates over Pd metal cations in ALD deposited AlOx and HfOx. However, in sputtered SiOx, Pd cation transport was revealed, accompanied by the formation of nano-crystalline Pd filament(s) in the junctions. Based on these observations, memristors with reversible and repeatable switching were obtained by using Pd doped SiOx as the switching material.
- This article is part of the themed collection: Electrochemical processes at the nanoscale: from fundamentals to applications


 Please wait while we load your content...
Please wait while we load your content...