Synthesis and molecular structure of arene ruthenium(ii) benzhydrazone complexes: impact of substitution at the chelating ligand and arene moiety on antiproliferative activity†
Abstract
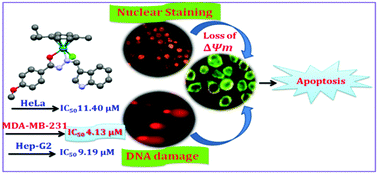
A convenient method for the synthesis of ruthenium(II) arene benzhydrazone complexes (1–6) of the general formula [(η6-arene)Ru(L)Cl] (arene-benzene or p-cymene; L-monobasic bidentate substituted indole-3-carboxaldehye benzhydrazone derivatives) has been described. The complexes have been fully characterized via elemental analysis, IR, UV-vis, NMR and ESI-MS spectral methods. The solid-state molecular structures of the representative complexes were determined using a single-crystal X-ray diffraction study and the results indicated the presence of a pseudo octahedral (piano stool) geometry. All the complexes were thoroughly screened for their cytotoxicity against human cervical cancer cells (HeLa), human breast cancer cell line (MDA-MB-231) and human liver carcinoma cells (Hep G2) under in vitro conditions. Interestingly, the cytotoxic activity of complexes 3, 4 and 6 is much more potent than cis-platin with low IC50 values against all the cancer cell lines tested. Furthermore, the mode of cell death in the MDA-MB-231 cells was assessed via AO–EB staining, Hoechst 33258 staining, flow cytometry and comet assay. Furthermore, the results of Western blot analyses suggest that complexes 3 and 6 accumulate preferentially in the mitochondria of MDA-MB-231 cells and induce apoptosis via mitochondrial pathways by up-regulating p53 and Bax, and down-regulating Bcl-2.

 Please wait while we load your content...
Please wait while we load your content...