Development of porphyrin syntheses
Abstract
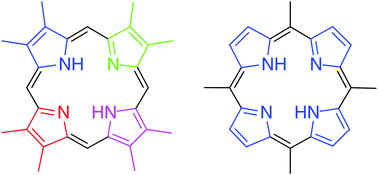
Hans Fischer (Munich, Germany) and S. Fergus MacDonald (Ottawa, Canada) devised efficient porphyrin syntheses based on condensation reactions of dipyrromethenes (Fischer) or dipyrromethanes (MacDonald). These historically significant processes and subsequent developments are discussed, along with monopyrrole tetramerizations that give 5,10,15,20-tetra-arylporphyrins, to show that the art of porphyrin synthesis continues to flourish.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...