Synthesis, structural characterization, electrochemical behavior and anticancer activity of gold(iii) complexes of meso-1,2-di(1-naphthyl)-1,2-diaminoethane and tetraphenylporphyrin†
Abstract
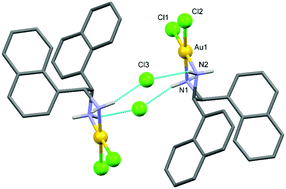
Three gold(III) complexes, [Au(npen)Cl2]Cl·2H2O (1), [Au(npen)2]Cl3 (2) and [Au(TPP)]Cl (3) (npen = meso-1,2-di(1-naphthyl)-1,2-diaminoethane, TPP = meso-tetraphenylporphyrin) have been synthesized and characterized using elemental analysis, IR and NMR spectroscopy, and one of them (1) by X-ray crystallography. The structure of 1 consists of a [Au(npen)Cl2] complex ion, a chloride counter ion and two water molecules. The gold atom in the complex ion adopts a distorted square planar geometry. The interactions of 1 and 2 with L-tyrosine, glutathione and lysozyme were studied electrochemically. The electrochemical measurements indicated that gold(III) remained stable and did not undergo reduction upon interaction with proteins. The in vitro cytotoxic properties of the complexes as well as of cisplatin were evaluated on three human cancer cell lines, A549 (lung cancer cells), MCF7 (breast cancer cells) and HCT15 (colon cancer cells) using MTT assay. The results indicated that the prepared gold(III) complexes were more potent than cisplatin in inhibiting the growth of the selected cancer cells. The IC50 data revealed that complex 3 was the most effective antiproliferative agent.

 Please wait while we load your content...
Please wait while we load your content...