Organometallic derivatives of natural products: dicobalt hexacarbonyl complexes of geranyl-alkynes†
Abstract
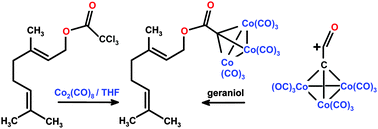
Treatment of methyl geranyl ether with diiron nonacarbonyl leads to hydrogen migration to form previously unknown E and Z 1-methoxy-3,7-dimethyl-1,6-octadiene in low yield. Sodium propargyl alkoxide and geranyl bromide yield propargyl geranyl ether, 13; subsequent reaction with dicobalt octacarbonyl and then bis(diphenylphosphino)methane furnishes the corresponding alkyne–Co2(CO)4(dppm) tetrahedral cluster, 16. Reaction of geranylacetone with phenylethynyl-lithium, and then with Co2(CO)8, forms (1-phenyl-3,7,11-trimethyldodeca-6,10-dien-1-yn-3-ol)Co2(CO)6, 19. The carbynyltricobaltnonacarbonyl clusters RC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CCo3(CO)9, where R = geranyl, 23, or farnesyl, 25, are preparable in very good yield either by reaction of the appropriate alcohol with trichloroacetyl chloride and then Co2(CO)8, or by reaction with the metal-stabilized acylium ion [Co3(CO)9C
O)CCo3(CO)9, where R = geranyl, 23, or farnesyl, 25, are preparable in very good yield either by reaction of the appropriate alcohol with trichloroacetyl chloride and then Co2(CO)8, or by reaction with the metal-stabilized acylium ion [Co3(CO)9C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]+, 24. Potential use of these (η2-alkyne)dicobalt complexes in Pauson–Khand or Nicholas cyclizations is discussed.
O]+, 24. Potential use of these (η2-alkyne)dicobalt complexes in Pauson–Khand or Nicholas cyclizations is discussed.

 Please wait while we load your content...
Please wait while we load your content...