Solvent-less method for efficient photocatalytic α-Fe2O3 nanoparticles using macromolecular polymeric precursors†
Abstract
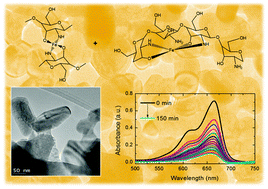
We report a method for solvent-less growth of single crystalline hematite Fe2O3 nanoparticles from metal-containing polymeric macromolecular complexes, and demonstrate their efficient photocatalytic degradation of persistent cationic dye pollutants under visible light. Macromolecular complexes such as chitosan·(FeCl2)y, chitosan·(FeCl3)y, PS-co-4-PVP·(FeCl2)y and PS-co-4-PVP·(FeCl3)y with controlled polymer : metal molar ratios of 1 : 1 and 5 : 1 were prepared by single reaction of the respective polymers and iron chloride salts in CH2Cl2. The stable insoluble compounds were characterized by elemental analysis, infra-red spectroscopy, EPR and diffuse reflectance spectroscopy, and confirm Fe salts with degrees of coordination of ∼60–70%. Pyrolysis of these macromolecular precursors under air and at 800 °C forms networked Fe2O3 nanoparticles, whose volumetric density, size and shape is controlled by the metal content and the nature of the macromolecular complex (chitosan or PS-co-4-PVP). For both polymers, the 1 : 1 molar ratio precursor produces nanoparticles ranging from 10–200 nm with a moderate superparamagnetic behavior and optical bandgap marginally larger than bulk Fe2O3. A matrix-incubated formation mechanism involving the carbonization of the organic matter, forming voids within the macromolecular complex wherein the Fe centres coalesce, oxidize and crystallize into nanoparticles is also proposed. The hematite Fe2O3 nanoparticle materials demonstrate very efficient photocatalytic degradation of persistent water pollutants such as the cationic dye methylene blue. The nanoparticulate material obtained from chitosan·(FeCl2)y 1 : 1 under the simulated sunlight (full visible spectrum) irradiation provides high rate degradation of MB by 73% in 60 min and >94% after 150 min, measured at 655 nm.

 Please wait while we load your content...
Please wait while we load your content...