Two energetic complexes incorporating 3,5-dinitrobenzoic acid and azole ligands: microwave-assisted synthesis, favorable detonation properties, insensitivity and effects on the thermal decomposition of RDX†
Abstract
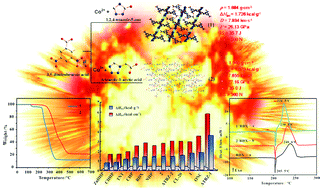
Two new energetic complexes, Co(TO)2(DNBA)2(H2O)2 (1) and Cu(TZA)(DNBA) (2) (TO = 1,2,4-triazole-5-one, HTZA = tetrazole-1-acetic acid, HDNBA = 3,5-dinitrobenzoic acid), were synthesized using a microwave method with high yields. Single crystal X-ray diffraction revealed that 1 possess a 3D supramolecular structure with mononuclear units and 2 has a 2D supramolecular architecture with 1D chains. Thermoanalysis demonstrated that the two complexes have good thermostability, up to 238 °C for 1 and 270 °C for 2. The non-isothermal kinetics for exothermic processes of the complexes were studied using Kissinger's and Ozawa's methods. The calculated detonation properties and sensitivity tests showed that both complexes could be used as potential explosives. In addition, the two complexes were explored as combustion promoters to accelerate the thermal decomposition of RDX (1,3,5-trinitro-1,3,5-triazinane) using differential scanning calorimetry.

 Please wait while we load your content...
Please wait while we load your content...