Boron templated synthesis of a BODIPY analogue from a phthalocyanine precursor†
Abstract
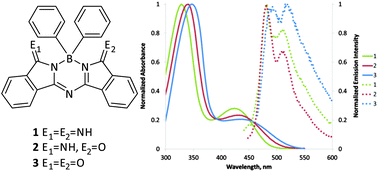
The synthesis of aza(dibenzopyrro)methenes (ADBM) using aryl boron compounds as templates is described. The resultant half phthalocyanine BPh2 compounds (1–3) are structurally related to the BODIPY class of fluorophores and were isolated with terminal imine, terminal oxo, or mixed imine-oxo heteroatom positions. Compounds 1–3 were investigated for their absorption/emission properties, and large stokes shifts of ∼50 nm were observed for fluorescence emission. The electronic structures of 1–3 were probed by TD/DFT methods.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...