Selective complexation of di-n-hexylammonium salts by tailed porphyrin host†
Abstract
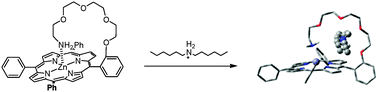
Novel types of tailed porphyrins have been synthesized and characterized. The tail was coordinated intramolecularly to zinc porphyrin, leading to the formation of a cavity. In the cavity, tailed porphyrins formed a complex with di-n-hexylammonium salts. The binding affinities of the tailed porphyrins were studied by 1H NMR and UV-visible titration experiments. The selectivity of complexation was attributed to the induced fit and lipophilic interactions.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...