Unsaturation in binuclear heterometallic carbonyls: the cyclopentadienyliron manganese carbonyl CpFeMn(CO)n system as a hybrid of the Cp2Fe2(CO)n and Mn2(CO)n systems†
Abstract
The experimentally known CpFeMn(CO)7 system as well as the related unsaturated CpFeMn(CO)n systems (n = 6, 5) have been investigated by density functional theory. For CpFeMn(CO)7 unbridged and doubly bridged structures with a heteronuclear Fe–Mn single bond lie within ∼5 kcal mol−1 of each other with the experimentally known unbridged structure being the lower energy structure. The three lowest-energy unsaturated CpFeMn(CO)6 structures, lying within ∼1 kcal mol−1 of each other, have very diverse structural features. The lowest-energy CpFeMn(CO)6 structure contains an unusual three-center two-electron C–H–Mn bond involving an agostic hydrogen of the Cp ring. Another such structure is a triply bridged triplet CpFe(μ-CO)3Mn(CO)3 closely related to the experimentally known Cp2Fe2(μ-CO)3 having a central 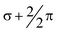 Fe
Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn double bond containing the two unpaired electrons of the triplet spin state. The third low-energy CpFeMn(CO)6 structure is a CpFe(CO)Mn(CO)4(η2-μ-CO) structure with a four-electron donor bridging η2-μ-CO group similar to that found in the lowest energy Mn2(CO)9 structure. The lowest-energy structure of the even more unsaturated CpFeMn(CO)5 has a short Fe
Mn double bond containing the two unpaired electrons of the triplet spin state. The third low-energy CpFeMn(CO)6 structure is a CpFe(CO)Mn(CO)4(η2-μ-CO) structure with a four-electron donor bridging η2-μ-CO group similar to that found in the lowest energy Mn2(CO)9 structure. The lowest-energy structure of the even more unsaturated CpFeMn(CO)5 has a short Fe![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn distance of only ∼2.2 Å suggesting a formal triple bond. Higher energy CpFeMn(CO)5 structures have a four-electron donor η2-μ-CO group in addition to a formal Fe
Mn distance of only ∼2.2 Å suggesting a formal triple bond. Higher energy CpFeMn(CO)5 structures have a four-electron donor η2-μ-CO group in addition to a formal Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn double bond. The CpFeMn(CO)5 structure in which the oxygen atom of the η2-μ-CO group is bonded to manganese lies ∼9 kcal mol−1 in energy below the isomeric structure in which the oxygen of the η2-μ-CO group is bonded to iron.
Mn double bond. The CpFeMn(CO)5 structure in which the oxygen atom of the η2-μ-CO group is bonded to manganese lies ∼9 kcal mol−1 in energy below the isomeric structure in which the oxygen of the η2-μ-CO group is bonded to iron.


 Please wait while we load your content...
Please wait while we load your content...