Oxaazamacrocycles incorporating the quinoline moiety: synthesis and the study of their binding properties towards metal cations†‡
Abstract
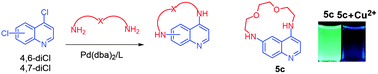
Novel dioxa- and trioxadiazamacrocycles have been synthesized by the Pd(0)-catalyzed amination of 4,6- and 4,7-dichloroquinolines with linear di- and trioxadiamines. Macrocyclization reaction was shown to be more successful for 4,6-dichloroquinoline, providing the corresponding macrocycles with yields of up to 32%. 4,6-Di(2-methoxyethylamino)quinoline was obtained in 88% yield for comparative studies. The synthesis of macrocycles comprising two 4,7-disubstituted quinoline moieties and two oxadiamine linkers has been accomplished. The binding properties of 4,6-diamino derivatives of quinoline have been studied with 17 metal cations using UV-vis and fluorescence spectroscopy. UV, fluorescence, and NMR spectral data demonstrated the formation of complexes of different compositions depending on the nature of the ligand and the metal cation. One of the macrocycles (5c) was shown to be applicable as a selective fluorescent and colorimetric chemosensor for Cu(II). Macrocyclic ligands 5 clearly showed a different behavior in the presence of metal cations compared to the non-cyclic derivative 10.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...