Hydrothermal synthesis, structures and properties of two uranyl oxide hydroxyl hydrate phases with Co(ii) or Ni(ii) ions†
Abstract
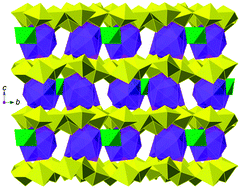
Two new iso-structured uranyl oxide hydroxyl hydrate (UOH) phases with the incorporation of cobalt(II) or nickel(II) ions have been synthesised under hydrothermal conditions and structurally characterised. Both K4Co(OH)3(H2O)9[(UO2)12(O)7(OH)13] (1) and K4Ni(OH)3(H2O)9[(UO2)12(O)7(OH)13] (2) have two-dimensional (2D) polymeric uranyl oxohydroxyl layers with either potassium and hydroxyl cobalt(II) (1) or potassium and hydroxyl nickel(II) (2) ions between layers via uranyl–cation interactions. This work highlights the feasibility of making new UOH phases via a hydrothermal route at relatively higher solution pHs. It also demonstrates that other transition metal ions which are readily available in the environment may also be incorporated into such UOH phases during the natural weathering of uraninite as well as during the storage and disposal of spent nuclear fuels.

 Please wait while we load your content...
Please wait while we load your content...